|
|
中国有色金属学报 |
|
|
|
||
|
Phase and structure of
polycrystal Tian Yanwen(田彦文),
Ma Ping(马 平), Zhang Xin(张 新), Zhai Yuchun(翟玉春) Abstract: The phase
and the structure of polycrystal material, which was prepared by calcining
the co-precipitated fluoride powder of Sr and La with a ratio of 0.69∶0.31
mixed with SrS powder, were investigated by X-ray diffraction and electron
probe scanning. In the materials, three phases were found, they are Sr0.69La0.31F2.31
solid solution phase with a pure SrF2 cubic structure whose
lattice constant is 5.843 Document
code: A 1 INTRODUCTION In order to make the solid electrolyte sensor used in
measuring sulfur activity in metallurgical process, many investigations were
carried out[1~3]. The research on measuring sulfur activity in the
atmosphere at the temperature below 500 ℃ was comparatively successful[4],
but the researches on measuring sulfur activity in metallurgical melts at
high temperatures were developed very slowly. No suitable solid electrolyte
can be used in measuring sulfur activity in the melts at present, and the
researches on this solid electrolyte are still in the beginning. 2 PREPARATION OF SAMPLES First, the co-precipitated and dehydrated fluoride
powder of Sr and La with a ratio of 0.69∶0.31 was mixed with SrS. And then
the mixture was molded by isostatic pressure at 280 MPa. After that, the
molded mixture was-dehydrated for 2.5 h at 450 ℃ and calcined for 7 h at 800
℃ in the approximately vacuum equipment with a lower oxygen activity.
Finally, three kinds of samples were obtained as following: sample A without
SrS, sample B and C mixed with SrS in 3%(mass fraction) and 5% (mass
fraction) respectively. 3 RESULTS OF XRD AND
DISCUSSION It was revealed by XRD analysis that in this experiment
the prepared powder SrF2, LaF3 and SrS samples are pure
SrF2 with fluorite cubic structure, pure LaF3 with
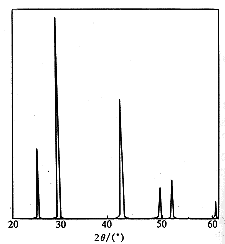
hexagonal structure and pure SrS with NaCl cubic structure respectively, and
no phase change happens. Their XRD spectra are shown in Figs.1, 2 and 3.
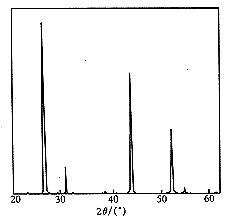
Fig.1 XRD spectrum
of SrF2
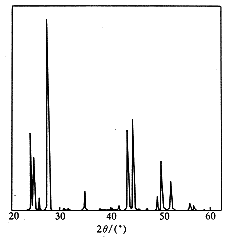
Fig.2 XRD spectrum
of LaF3
Fig.3 XRD spectrum
of SrS The XRD spectra of samples A, B and C are shown as
Fig.4.
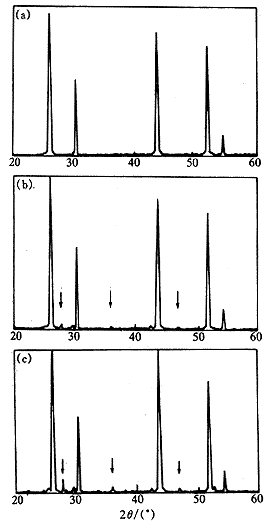
Fig.4 XRD spectra
of sample A, B and C It can be found that both positions and shapes of main
diffraction peaks in the spectra of the three samples were correspondence
match. Comparing with the main peaks of the pure SrF2 spectrum
(seeing Fig.1), those of samples A, B, C have the same shape, but the
positions of main diffraction peaks of these samples are a bit shift to the
low angle, indicating an increased lattice constant. When comparing with the
main peaks of the pure LaF3 spectrum (seeing Fig.2), those of
samples A, B, C have inconsistent shapes and positions, it indicates that the
samples A, B and C take SrF2 cubic structure.
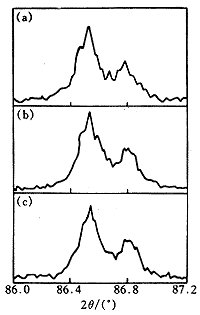
Fig.5 Amplified
XRD spectra of The Kα1 and Kα2 peaks of the
three samples did not split and they are correspondence match. So it can be
further proved that in the three samples all La and Sr atoms take the same
position in the lattice and form a solid solution with SrF2 cubic
structure. Table 1 d value
and relative intensity of |
|
Diffraction |
Sample
B |
Sample
C |
||
|
d/ |
I/I0 |
d/ |
I/I0 |
|
|
1 |
3.180 |
2 |
3.186 |
3 |
|
2 |
2.482 |
1 |
2.483 |
1 |
|
3 |
1.932 |
1 |
1.933 |
1 |
|
It was
revealed by computer index that the undetermined peaks match the value of MnI2
standard card. But in this research no Mn element and I element were
introduced into the samples. By far, the undetermined peaks are still XRD
spectra of an unknown phase. 4 RESULTS OF EPS AND
DISCUSSION Morphology observation and element scanning on fresh
fracture face of block samples A, B and C were carried out by EPS. Because
the element whose atomic number is smaller than 10 can not be detected by the
selected device, only La, Sr and S were measured by EPS.
Fig.6 EPS images
of samples with SrS Combined with the spectra of XRD, Fig.6 shows that, in
the area where there is no S element, La element and Sr element coexist and
distribute uniformly, forming a very uniform solid solution phase. In the
area where there was much S element, Sr element but no La element existed,
and this reveals that there existed the SrS phase. In the area where there
was less S element, La element and Sr element coexisted, indicating an
unknown phase produced by the interaction of SrS and co-precipitated powder.
At present the unknown phase can not be determined. 5 CONCLUSIONS (1) In the Sr0.69La0.31F2.31+SrS
(with SrS content no more than 5%) polycrystal material, Sr0.69La0.31F2.31
solid solution matrix phase, small amount SrS phase and an unknown phase
which is produced by the interaction of SrS and co-precipitated powder are
found. Three diffraction peaks of the unknown phase are also obtained.
Moreover, the content of SrS phase and unknown phase increases with
increasing SrS content. ① Project 901450 supported by the Doctoral Foundation of
the Education Ministry of China REFERENCES 1 Worrell W L and Liu Q G. US Patent 4428770. Received Nov. 13,
1998; accepted Apr. 7, 1999 |