Deterministic
Chaos in the Cardiac System
Franηois
Roy
MAT335
Dr. Randall Pyke
April 7th, 2002
Contents
Preface
3
Introduction
3 Feedback Mechanisms
4
Dynamic Systems and Chaos
.. 6
Unstable Periodic Orbits
. 8
Time Scales
. 10
Fractal Dimension
... 12
Chaos Control
. 16
Conclusion
.. 18
References
.. 20
Preface
In recent years,
cardiovascular diseases have posed major threats to human life. When studying
cardiac diseases and their possible therapies, it is important to understand
the pathological (diseased) changes that occur in the cardiac system. Healthy
cardiac muscles contract periodically and are stimulated by electrochemical
waves that spread throughout the heart. Thus, analysis of heart rates and electrocardiograms
(ECGs) has proven to be a useful method for quantifying the efficiency of the
heart. Recent studies have suggested that complex feedback mechanisms in
the cardiovascular system are responsible for the underlying heart rate
behaviour and heart rate variability (West et al., 1999). The cardiovascular
system is governed by feedback mechanisms that regulate blood pressure, blood
flow, blood ion concentrations, body temperature, and so on. Thus, the
behaviour of the heart is generated from feedback systems and this strongly
suggests that the heart behaves as a dynamic system.
Introduction
The hypothesis that
the heart behaves as a dynamic system can be validated if instances of chaotic
behaviour can be observed via the analysis of heart rates and
electrocardiograms (ECGs). Fortunately, using the proper tools from dynamics
and chaos, strong evidence of chaos has been observed. Thus, it is safe to
assume that the variability in cardiac behaviour is generated via nonlinear
dynamics. In this sense, the deterministic behaviour generated from the feedbacks
can also yield a corresponding time series or data set similar to that
of other dynamic systems including the logistic equation. When plotted
properly, the points of the time series can generate graphs or pictures with a
high degree of structure that would otherwise not occur from completely random
series. Deterministic chaos further exhibits sensitive dependence on the
initial conditions of the system. Thus, by using the Lyapunov exponent,
a measure of divergence from initial conditions, further evidence of chaos has
been observed in the cardiac system (Hagerman et al., 1996). Evidence of unstable periodic orbits
(UPO) embedded in the cardiac rhythms has been discovered and can be used
to describe the intrinsic properties of the attractors (Narayanan et al.,
1997). The number of unstable periodic orbits found on the attractors of four
different patients yields strong evidence that suggests that unstable periodic
orbits can be used to quantify cardiac behaviour and diagnose the state of
health of the cardiac system. It was also observed that time scales play
an important role in the behaviour of the cardiac system (West et al., 1999).
The appearance of highly periodic heart rates, similar to sine waves, and
reduced time scales in cardiac diseased states support the notion that disease
or cardiac failure is intrinsically linked to a loss in complexity. Self-similarity
in the dynamic structure of heart rates, as seen on a heart rate vs time plots,
reveals evidence of fractal dimension embedded in the behaviour of the cardiac
system (Wagner et al., 1998). Recent algorithms attempting to calculate the fractal
dimension of the heart rates have been developed to further improve the
efficiency of measuring these abstract dimensions. These algorithms include the
correlation dimension and the short-term fractal-scaling exponent.
Recent application and development of chaotic models using nonlinear tools have
allowed researchers to control the chaos of the cardiac system. Garfinkel et
al. (1995) successfully controlled chaos in arrhythmic (irregular) heart rates
by electronically perturbing heart rate contractions via a similar method
outlined by Ott et al. (1990). This further suggests that the cardiac system
behaves chaotically and can be controlled using the tools from nonlinear chaos.
Thus, this paper explores the nonlinear dynamics of the cardiac system along
with the nonlinear dynamical measures used to quantify and diagnose its
behaviour.
Feedback Mechanisms
Rhythmic changes in
heart rate have indicated that heart rate is not constant but varies
significantly over time even in the absence of physical and mental stress.
Analysis of heart rate oscillatory behaviour reveals a broad, noise-like
variability over a large span of frequencies and suggests that the irregular
variability and behaviour is due to nonlinearities in the control system of the
cardiac network (Wagner et al., 1998).
The specific mechanisms underlying heart rate behaviour and heart rate
variability are certainly not very clear. However, strong evidence suggests
that the heart rate is regulated by a complex feedback control mechanism involving
closed control systems regulated via positive and negative feedbacks (West et
al., 1999). The cardiovascular system is governed by feedback mechanisms that
regulate blood pressure, blood flow, blood ion concentrations, body
temperature, and so on. For example, if blood flow to the brain is
significantly decreased, pressure receptors in the brain send signals to the
heart demanding increased blood flow. The heart must respond to the brains
demands by altering its current behaviour and increase its cardiac output. This
example suggests that feedback or iteration processes form much of the
backbone of cardiac behaviour in the body. As many feedbacks interact
simultaneously on the cardiac system, it is clear that the heart needs complex
integrating behaviour to respond to the bodys needs. Complexity in the
feedback control indicates that the control system is in itself nonlinear.
Current evidence suggests that the observed heart rate variability time series
generated from nonlinear dynamics may be chaotic and deterministic in nature
(West et al., 1999).
Dynamic Systems and
Chaos
Garfinkel et al. (1995) attempted to discover
the deterministic order and behaviour in the heart rate time series.
Deterministic order implies that each point in a time series is
generated by the previous point via subsequent iterations. An example of this
is the standard logistic equation f(x) = ax(1-x) in which each point in
the time series is generated via xt+1 = f(xt). Common irregularities
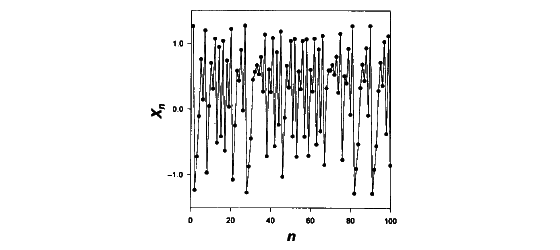
in a time series of data points x1, x2, x3,...,
xn are shown in figure 1 and can easily be thought to behave
randomly. In order to gather evidence of deterministic behaviour from an
arbitrary time series, it is often useful to look at the data from a different
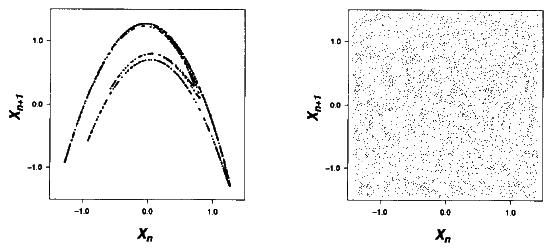
perspective. By plotting the points of a time series on an xn+1
vs xn plot; pictures can often be generated with a high
degree of structure called
|
|
|
Figure 1: Iteration of 100 points x1,
x2, x3, x4 x5,
, x100
using the Hιnon function (Garfinkel et al., 1995). |
the attractor. The contrast between a
deterministically generated times series and a random times series on a xn+1
vs xn plot can clearly be seen in Figure 2(a) and Figure 2(b)
respectively. The high degree of structure in figure 2(a) composed of
the elliptical cloud is certainly not what the random time series in Figure
2(b) could have generated. The presence of nonrandom structure in such a plot
is generally a good diagnosis of deterministic chaos. Deterministic chaos
further exhibits a number of characteristics that distinguish it from periodic
or random behaviour. In particular, as seen for the logistic equation, dynamic
systems have sensitive dependence on initial conditions. Thus, small
changes in initial conditions have large pronounced effects on the behaviour of
the system at some point in the future (Hagerman et al., 1996). This can often
be manifested as divergence of adjacent trajectories that diverge widely as
time or iteration evolves and can be measured via the Lyapunov exponent.
Hagerman et al. (1996) discovered that by disrupting the behaviour of the
cardiac system, the value of the largest Lyapunov exponent could be reduced. This further confirms the idea that the
cardiac system
|
|
||
|
behaves chaotically and stresses the importance
of using nonlinear dynamics as the tool for understanding the behaviour of the
cardiac system.
Unstable Periodic
Orbits
Further evidence
suggests that chaos is inherent of the physiological control of heart rate via
the discovery of unstable periodic orbits (UPO) in the cardiac rhythms.
Narayanan et al. (1997) discovered that the periodicity and distribution of the
orbits on the chaotic attractor are indicative of the state of health of the
cardiac system. Unstable periodic orbits can be detected and extracted from
chaotic attractors constructed from measured electrocardiograms and their
importance arises from the properties of the attractors. In this sense, the intrinsic properties of
the attractor can be expressed in terms of its unstable periodic orbits.
Unstable periodic orbits can often be observed on the attractors of
three-dimensional xn+2 vs xn+1 vs xn
plots and resemble loops. Attractors can themselves be created from heart rates
and ECGs, but only using an appropriate delay time t (Narayanan et al., 1997). It is certainly more
difficult to produce an attractor from ECGs since the time series cannot be
obtained from a single equation such as the logistic equation. However, using a
delay time t, it is possible to represent x1, x2, x3,...,
xn with x(t), x(t + t), x(t + 2t),
, x(t + (n - 1)t) respectively and a corresponding attractor
can subsequently be created. This method has also been implemented in another
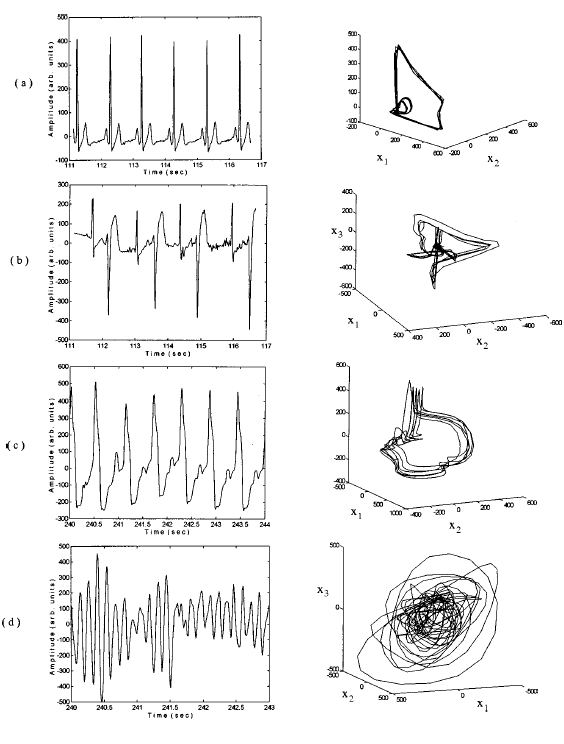
one of this years MAT335 projects. Figures
3(a)-3(d) show ECG time series and three-dimensional views of the chaotic
attractor for typically healthy and pathological diseased cases such as
premature ventricular contraction (PVC), ventricular tachy arrhythmia (VTA),
and ventricular fibrillation (VF) respectively. Importantly, the ECGs for the
normal case can
|
|
|
Figure 3: ECG time series and
attractors for (a) normal, (b) PVC, (c) VTA, and (d) VF cases respectively. x1,
x2, and x3 represent x(t), x(t + t), and x(t + 2t) respectively
where x is the time series and t is the delay time (Narayanan et al., 1997). |
be distinguished qualitatively from the others,
but behaviour and structure of the chaotic attractors seem to yield differences
that are more pronounced. Thus, by finding and extracting the unstable periodic
orbits from the four attractors, Narayanan et al. (1997) discovered distinct
quantitative differences for each of the cases. Figures 4(a) and 4(b) represent
a comparison of the unstable periodic points for each of the five cases. The
normal attractor of the cardiac systems is characterised by three or four
unstable periodic orbits with typical periodicity and intensity where the
strongest unstable periodic orbit is found to be in the range of m
values 85-95 in arbitrary units and has a basic period of 0.94-1.05 seconds
(Narayanan et al., 1997). In contrast to the healthy patients, the number of
unstable periodic orbits varied significantly for the other four patients. This
evidence further suggests that the cardiac system has been generated via
nonlinear dynamics and often shows striking characteristics of chaos.
|
|
||
|
Time Scales
Intrinsically linked
to the chaotic attractors of the cardiac system produced from ECGs, time scales
play an important role in the behaviour of the cardiac system. The erratic
behaviour of the ECG time series is a consequence of deterministic, nonlinear,
and dynamic interactions between the biological components of the cardiac
system (Ott et al., 1993). West et al. (1999) argue that the cardiac control
system has innate properties that enable it to respond to a dynamic biological
environment via scaling. The idea of scaling will become clearer later
on. The chaotic motion or fluctuations in the time series include unstable
periodic orbits that are used to move the system from one orbit to the next,
thus giving the impression of randomness. Nevertheless, these apparent random
fluctuations are what contain the information about the system. West et al.
argue that this apparent random fractal time series in a biological
context is the direct consequence of feedback control that operates over
multiple time scales. In particular, heart rate interval fluctuations indicate
that there exists long-term memory embedded in the randomness. This long-term
memory is evident via the presence of an attractor as seen in Figure 2(a) as
compared to randomness or the lack of memory in Figure 2(b). These long-term
correlations or memories observed in the heart rate variability suggest that,
although different regulatory mechanisms may act independently on different
time scales, their dynamic effects on heart rate may be linked together via
scaling. Thus, heart rate regulation would certainly be effected if one of the
feedback mechanisms was impaired. Goldberger (1996) suggests that the cardiac
system is self-organised in such a way that it has a characteristic time scale
which gives it more plasticity and increases its ability to behave according to
the feedbacks. The appearance of highly periodic dynamics or behaviours in many
disease states is a compelling example which supports the notion that disease
or cardiac failure is intrinsically linked to complexity loss and loss of
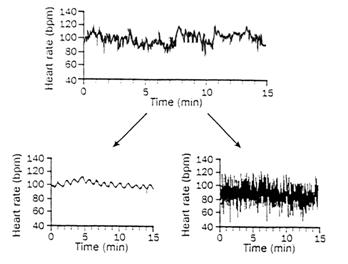
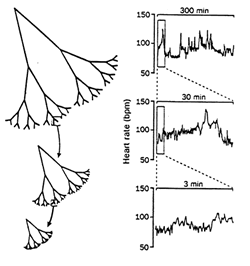
time scales. Figure 5 represents examples of relative time scales of
individuals in different cardiac
|
|
|
Figure 5: Healthy dynamics showing multiple scales (top),
pathological breakdown to single scale sinus-rhythm (bottom left), and
ventricular fibrillation randomness (Goldberger, 1996). |
states. The normal and healthy dynamics show multiple
time scales and fractal behaviour whereas the single scaled sinus-rhythm heart
represents severe heart failure. The behaviour of atrial fibrillation is
characterized by uncorrelated randomness and certainly would not pass any of
the tests for deterministic chaotic as mentioned earlier. Thus, apparent
fluctuations present in chaos contain information about the system and, by
attempting to determine the information embedded in the time-series, it can
prove to be beneficial to use the tools from chaos to analyse the cardiac
system.
Fractal Dimension
Furthermore, evidence has indicated that
scaling properties of heart rate behaviour can be quantified by the use of
fractal dimension. Schematic representations of self-similar structure of
dynamics in figure 6 suggest the importance of fractal dimension for
understanding and analysing the heart rate fluctuations on different time
scales. Importantly, all three of the graphs have a somewhat irregular
appearance similar to that of coastlines or mountain ranges. Mechanically,
Goldberger (1996) suggests that self-similarities in the heart rate serves a
physiological function of rapid and efficient transport over a complex and
spatially distributed system. Mathematically, fractal dimension is used
to give a dimension of the statistical measure of the geometry of a cloud of
points and can be assigned to any arbitrary data set (Wagner et al., 1998).
Attractors produced from cardiac systems have revealed non-integer fractal
dimensions (Mδkikallio et al., 2001). Standard algorithms for estimating
fractal dimension require very large data sets (>10 000), thus newer
algorithms have been developed to improve on data analysis. Conventional box
counting methods can certainly be used to calculate the fractal dimension of
curves generated from heart rates, however algorithms that are more efficient
can operate with shorter sequences of points (Wagner et al., 1998). One of
these algorithms calculating the correlation dimension is used to
describe the complex structure of the cardiac attractor by approximating its
fractal dimension (Hagerman et al., 1996). The correlation dimension algorithm
proposed by Grassberger and Procaccia
|
|
|
Figure 6: Representation of self-similar structure and
self-similar dynamics. The tree-like fractal has self-similarity such as
fractal geometry in nature. The heart rate generates fluctuations of multiple
time scales that are statistically self-similar (Goldberger, 1996). |
(1983) attempts to extract information from the
heart rate time series using another similar time delay t as outlined earlier (i.e. representing x1,
x2, x3,..., xn with x(t), x(t + t), x(t + 2t),
, x(t + (n - 1)t)). By measuring the distance between every
pair of points on the attractor, the correlation dimension can be calculated
as:
![]()
where H(x) represents a heaviside step function. Via a bilogarithmic
plot of C(N, r) vs r, the correlation dimension is an estimate of the slope of
the plotted curve (Roa et al., 2001). The correlation dimension does not measure
the fractal dimension of the heart rate per se, but harnesses the
fractal-like structure or
behaviour of the heart vs time curves. Varying estimates of the cardiac correlation
dimension ranging from 3.6 to 5.2 by Babloyantz et al. (1988) to more recent
values from 2.1 to 3.2 by Casseloggio et al. (1995) have been calculated.
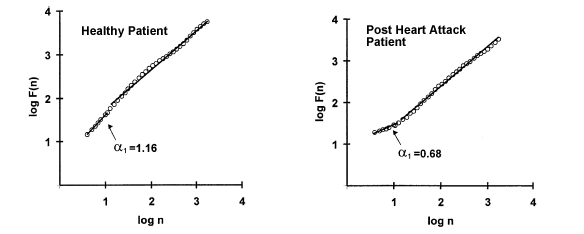
Recent algorithms exploring short-term fractal scaling analysis have
been used to further quantify the fractal-like properties of heart rates
(Mδkikallio et al., 2001). In this case, the root-mean square fluctuation of
the time series is measured in each observation window and is plotted against
the size of the window on a bilogarithmic scale. The mathematical reasoning
behind the use of these algorithms is somewhat unclear, however, useful
informative results have been obtained from these techniques. The short-term
fractal-scaling exponent a is calculated by approximating the slope of
the curve and can be seen in figure 8. Using the short-term fractal-scaling
exponent a1 measured in the
region where log(n) ![]() 1, Mδkikallio et al. (2001) suggest via the
Kaplan-Meier survival curve (figure 9) that short-term fractal-scaling exponent
a1 is a good indicator
of the state of health of the cardiac system. In the Kaplan-Meier survival
curves, higher short-term fractal-scaling exponents a1 had a significant survival advantage over the lower exponents a1. This suggests that there is an intrinsic link between fractal-like
dimension of heart rates and survival over time. Based on the data, I would
certainly hope that my own personal short-term fractal scaling would be at
least equal to 1. Thus, it is clear that fractal dimension, correlation
dimension, and the short-term fractal exponent all play an important role in
determining heart rate behaviour.
1, Mδkikallio et al. (2001) suggest via the
Kaplan-Meier survival curve (figure 9) that short-term fractal-scaling exponent
a1 is a good indicator
of the state of health of the cardiac system. In the Kaplan-Meier survival
curves, higher short-term fractal-scaling exponents a1 had a significant survival advantage over the lower exponents a1. This suggests that there is an intrinsic link between fractal-like
dimension of heart rates and survival over time. Based on the data, I would
certainly hope that my own personal short-term fractal scaling would be at
least equal to 1. Thus, it is clear that fractal dimension, correlation
dimension, and the short-term fractal exponent all play an important role in
determining heart rate behaviour.
|
|
|
Figure 8:
Typical example of short-term fractal-scaling exponent a1
.The post heart attack patient has a short-term fractal-scaling exponent a1 much smaller than a1 of the healthy subject
(Huikuri et al., 2001). |
|
|
|
Figure 9: Kaplan-Meier survival curve
of patients of various short-term scaling exponents a1. Data in this figure
correlates with figure 8 (Mδkikallio et al., 2001). |
Chaos Control
Now having explored
the cardiac system along with the tools used to model it, it is interesting to
discover some of the useful applications that have been developed. Ott et al.
(1990) showed that, by reducing the chaotic fluctuations within an acceptable
range, it is even possible to control chaos. Ott et al. used the properties of
the dynamics of stable and unstable manifolds of fixed points to control
the chaos of the system. Near one of the unstable fixed points, a state point
approaches the fixed point and then departs from it along preferred incoming
and outgoing directions called the stable and unstable manifolds or saddle
points. Ott et al. found a state point that wandered near a saddle point.
Subsequent iterations allowed the state point to wander away from the saddle
point along the unstable manifold. Thus, Ott et al. managed to use
perturbations to move the stable manifold to the location of the state point so
that the state point would essentially be sucked into the saddle point along
the stable manifold in the following iterations. The state point subsequently
approached the saddle point in the next iterations along the newly aligned
stable manifold. In this way, Ott et al. managed to confine the state point
|
|
|
Figure 10: Ott et al. (1990) control scheme. (a)
The nth iterate xn falls near the unstable
fixed point or saddle point x f(P 0). (b)
Perturbation dp moves fixed point and
corresponding stable and unstable manifolds. (c) The next iteration x n+1 falls in the stable
manifold. The state point is then attracted towards the saddle point along
the newly aligned stable manifold (Garfinkel et al., 1995). |
to a small neighbourhood of the saddle point
thus subsequently controlling the chaos (figure 10). This technique outlined by
Ott et al. has been extremely important for controlling the function of parts
of the nervous system and is currently being used to further understand and
model biological systems. Using similar techniques, Garfinkel et al. (1995)
were able to convert irregular arrhythmic heart rates in 8 of 11 cases into
normal periodic heart rates via the control of the hearts chaos. The
techniques used in these cases are no more complicated to understand than the
ideas from simple dynamics including the logistic equation. In essence,
the heart can be modelled as a simple pump. The behaviour of the pump
oscillates between two phases; a filling phase and an emptying phase. Thus, for
simplicity, the heart is modelled as a simple period-2 oscillator.
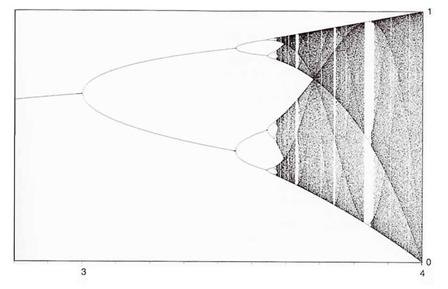
Therefore, its behaviour can be modelled by means of the standard logistic
equation f(x) = ax(1-x) in which the value of a lies in the
interval 3.01 to 3.4 and the behaviour of the heart will be governed by the
region in between the first and second
|
|
|
Figure
11: Final-state diagram for logistic equation and parameters a between
2.8 and 4 (Peitgen et al., 1992: 11.5) |
bifurcation on the final state diagram (Figure
11). Therefore, the behaviour of the heart will oscillate between the filling
phase and the emptying phase as predicted earlier. For a heart that beats
completely irregularly, this model predicts that the behaviour of the heart can
be characterised in the region of the final state diagram in which a ![]() 4. Thus, by controlling the value
of a for the logistic equation and the simplified heart model, it is
completely possible to control the behaviour of the heart. Often, electrical
currents can be used to perturb the system and control the value of a
and this technique is somewhat analogous to other techniques currently being
used to control chaos. Thus, it is clear that the application of chaos control
in biological systems will certainly prove to be useful for regulating the
behaviour of the cardiac system.
4. Thus, by controlling the value
of a for the logistic equation and the simplified heart model, it is
completely possible to control the behaviour of the heart. Often, electrical
currents can be used to perturb the system and control the value of a
and this technique is somewhat analogous to other techniques currently being
used to control chaos. Thus, it is clear that the application of chaos control
in biological systems will certainly prove to be useful for regulating the
behaviour of the cardiac system.
Conclusion
This paper explores
the nonlinear dynamics of the cardiac system along with the tools of chaos used
to analyse and diagnose its behaviour. Studies suggest that complex feedback
mechanisms in the cardiovascular system are responsible for the underlying
heart rate behaviour and heart rate variability (West et al., 1999). The
feedback mechanisms provide a complex control pattern that behaves
deterministically via nonlinear dynamics. Thus, deterministic chaos of the
cardiac system generated from the control systems yields a corresponding time
series that shows a high degree of structure that would otherwise not be
predicted from uncorrelated random points. Sensitive dependence on initial
conditions along with behaviour of the Lyapunov exponent has been useful for
characterizing the dynamic measure of the cardiac system (Hagerman et al.,
1996). The presence of unstable periodic orbits embedded in the cardiac rhythms
has also been used to quantify cardiac behaviour and diagnose the health state
of the cardiac system (Narayanan et al., 1997). The appearance of highly
periodic dynamics and reduced time scales in cardiac disease states supports the
notion that disease or cardiac failure is intrinsically linked to a loss of
complexity (West et al., 1999). Self-similarity in the dynamical structure of
heart rates also reveals evidence of fractal dimension embedded in the
behaviour of the cardiac system (Wagner et al., 1998). Evidence of the fractal
dimension in the cardiac system can also be seen via correlation dimension and
short-term fractal-scaling exponents implemented to improve the efficiency of
dimensional analysis of the cardiac system. Nonetheless, evidence suggests that
fractal dimension, correlation dimension, and the short-term fractal exponents
are all intrinsically linked to the behaviour of the cardiac system. Recent
applications and development of chaotic models using nonlinear tools from
Garfinkel et al. (1995) and Ott et al. (1990) have indicated that the nonlinear
systems can be controlled using the tools from chaos. Thus, it is clear that
improving knowledge and understanding of mathematical chaos will inherently
lead to an enhanced understanding of the complex behaviour of the cardiac
system.
References
Babloyantz,
A.; Destexhe, A. 1988. Biological Cybernetics. 58: 203.
Casaleggio,
Aldo; Braiotta, Stefano. 1997. Estimation of Lyapunov Exponents of ECG Time
Series: The Influence of Parameters. Chaos, Solitons & Fractals.
8(10): 1591-1599.
Casseloggio,
A.; Corana, A. 1995. Chaos, Solitons & Fractals. 5: 713.
Garfinkel,
A.; Weiss, J.N.; Ditto, W.L.; Spano, M.L. 1995. Chaos control of cardiac
arrhythmias. Trends in Cardiovascular Medicine 5(2): 76-80.
Goldberger,
Ary L. 1996. Non-linear dynamics for clinicians: chaos theory, fractals, and
complexity at the bedside. The Lancet. 347: 1312-1314.
Grassberger,
P.; Procaccia,
Hagerman,
Inger; Berglund, Margareta; Lorin, Mikael; Nowak, Jacek; Sylvιn, Christer.
1996. Chaos-related deterministic regulation of heart rate variability in time-
and frequency domains: effects of autonomic blockade and exercise. Cardiovascular
Research 31(3): 410-418.
Henon,
M. 1976. A two-dimensional mapping with a strange attractor. Communications
in Mathematical Physics. 50: 69-77.
Huikuri,
Heikki V.; Mδkikallio, Timo H. 2001. Heart rate variability in ischemic heart
disease. Autonomic Neuroscience. 90(1-2): 95-101.
Lampert,
Rachel J. 1996. Diurnal Variation of Heart Rate Fractal Dimension in Heart
Disease Patients. Journal of the
Mδkikallio,
Timo H.; Huikuri, Heikki V.; Hintze, Ulrik; Videbζk, Jorgen; Mitrani, Raul D.;
et. al. 2001. Fractal analysis and time- and frequency-domain measures of heart
rate variability as predictors of mortality in patients with heart failure. The
American Journal of Cardiology. 87(2): 178-182.
Meesmann,
M.; Boese, J.; et al. 1993. Demonstration of of 1/f fluctuations and white
noise in the human heart rate by the variance-time-curve: implications for
self-similarity. Fractals. 1: 312-320.
Narayana,
K.; Govindan, R.B.; Gopinathan, M.S. 1997. Unstable periodc orbits in human
cardiac rhythms. Physical Review E. 57(4): 4594-4603.
Ott,
E.; Grebogi, C.; Yorke, J.A. 1990. Controlling chaos. Physical Review
Letters. 64: 1196-1198.
Ott,
E. 1993. Chaos in Dynamical Systems.
Peitgen,
H.O. :Jurgens, H.; Dietmar, S. 1992 Chaos and Fractals: New Frontiers of
Science. Springer:
Rao,
R.K.A.; Yeragani, V.K. 2001. Decreased chaos and increased nonlinearity of heart
rate time series in patients with panic disorder. Autonomic Neuroscience:
Basic and Clinical. 88(1-2): 99-108.
Wagner,
C.D.; Persson, P.B. 1998. Chaos in the cardiovascular system: An update.
Cardiovascular Research 40(2): 257-264.
West,
B.J.; Zhang, R.; Sanders, A.W.; Miniyar, S.; Zuckerman, J.H.; Levine, B.D.
1999. Fractal fluctuations in cardiac time series. Physica A 270(3-4):
552-566.