









|
 |
| WEEK 1 - COLLECTING INSECTS: |
| Note: Additional information regarding insect collection can be found at the Entomological Society of Canada Website |
| EQUIPMENT YOU WILL BE GIVEN:
1. killing jar 2. ethyl acetate 3. collection box 4. insect pins 5. pinning block 6. glass vials |
    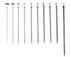  
1. 2. 3. 4. 5. 6. 7. |
| |
|
| METHODS OF CAPTURING INSECTS:
|
| 1. SWEEP-NETTING: (Good for catching a variety of plant-feeding, or resting insects) - Holding the handle firmly, rapidly skim your insect net across grasses and shrubs, in a horizontal, figure-8 motion, 3-5 times. This method of capture yields an amazing number and variety of insects in a short period of time. Sweep-netting different types of vegetation will increase the variety of insects that you will capture. NOTE: If possible, avoid sweep-netting in bushes that contain sharp thorns (such as blackberry) as these will snag and possibly damage your net. |
 |

|
2. BEATING: (Good for catching a variety of insects residing amongst tree branches) - Vigorously shake or hit a tree branch while holding a white piece of paper, or a white sheet underneath it. The impact will cause any insects on the branch or within the needles/leaves to fall onto the paper or sheet, which you can quickly fold and place directly into a killing jar. |
 |
| 3. AERIAL NET: (Good for discriminately collecting insects in flight, particularly useful for dragonflies and butterflies that rarely land) - Successfully catching insects while in flight often requires patience. The ability of some flying insects to avoid capture (particularly damselfies and dragonflies) means that these species are usually captured more easily if the entomologist remains still, with the net ready to capture any insect that approaches close enough for capture. On the other hand, many flying insects (such as butterflies) are less able to avoid capture but fly in a chaotic pattern that makes capture difficult. It is not uncommon for an entomologist to chase after these insects and sweep them out of the air while on the run. |
 |
4. LIGHT TRAP: (Fairly fast and easy way to collect nocturnal, photo/thermo-taxic insects) - A light source (a porch light works perfectly for this) is left on during at dusk and/or night time. Insects which are attracted to the light/heat source will either land near it, or remain flying around it, from where you can use a killing jar or net to collect them. Optionally, a white sheet may be placed in front of the light source to enhance the glowing effect. |
 |
| |
|
| 5. PITFALL TRAP: (Low-maintenance method for collecting ground roving insects) - A pitfall trap is simply a smooth-sided cup dug into the ground. The cup should be inserted far enough into the ground that it's rim is flush with the ground's surface. In this way, most insects that walk near the cup's edge will fall in and become trapped. It is often preferable to place a small amount of water into the cup to prevent flying insects from escaping. NOTE: In rainy weather, an uncovered pitfall trap will quickly fill with water. To prevent this, it is common to cover the opening with a make-shift 'tent' created from leaves and local debris. | 
|
| 6. BERLESE FUNNEL: (Good for collecting small, soil/debris inhabiting insects) - Collect a handful of forest leaf litter or soil and place into a glass jar. Stretch some fine mesh or cloth over the mouth of a funnel and place the funnel (tip-down) into a jar containing a small amount of 90% ethanol. Invert your leaf-litter jar over the funnel's mesh and place a strong light source (light-bulb or flashlight) above the entire set-up (close enough to slowly heat the leaf litter from the top-down). As the light source heats and dries the leaf-litter, insects (and other organisms) within the litter will descend to the cool/moist area below, eventually falling through the funnel and into the ethanol, from which you can collect them. |

|
7. SCAT OR CARRION COLLECTION: (Method for discriminately capturing scat or carrion seeking insects) - If you happen across animal feces or a recently dead animal, chances are you will find one or more species of insect (dung or carrion beetles, for example) residing on or within your find. Most of these insects rarely leave the feces or carrion and can be removed using forceps. |

|
| |
|||
| 8. FLIGHT INTERCEPT TRAP: (A somewhat elaborate method for indiscriminately capturing flying insects) - Mesh or screen is suspended between two or so points (between two trees, for example) and intercepts insects in flight. Many insects that contact the net will fall to the ground, therefore placing pitfall traps below the net will often capture more insects. As well, some insects will fly upwards (toward sunlight) after contacting the net, therefore additional insects may be captured if the net is actually tapered at the top, funnelling insects into a net or jar placed at the apex. | 
|
9. AQUATIC INSECT CAPTURE: (A necessary method used to capture motile, aquatic insects) - Aquatic insects are most easily captured using a simple aquarium net or fine mesh. It is not unusual to find an abundance of insects swimming on or just below the water at the edges of ponds (where the water is warmer). As such, most aquatic insects can be captured from shore, without having to actually enter the water, but rubber boots or hip-waders will ensure the capture of those insects that are just out of reach or inhabiting muddy areas. If mud is collected, wash it through a sifter to separate out the insects. Or alternatively, dump the mud onto a waterproof white surface and examine for moving organisms. | 
|
| KILLING INSECTS: |
| 1. ETHYL ACETATE (KILLING FLUID): Captured insects can be easily killed by placing them into a jar containing a cotton ball or tissue paper soaked in a killing fluid such as ethyl-acetate. Most live insects trapped within a killing jar will die within 30 minutes so it is often the preferred method for catching insects in the field. However, insects killed in this way should be frozen or pinned as soon as possible (within 24 hours if possible) to prevent excessive drying prior to pinning. Pinning dried out insects usually results in damaged appendages. |
|

|
2. FREEZING: Another method of killing live insects is to place them into a container and place the container into a freezer. Although this method can take up to 12 hours to kill an insect, it usually prevents the insect from drying out prematurely. As long as frozen insects are not left in the freezer for more than a couple of weeks, they should be soft and pliable when thawed. Of course, it is unlikely that a freezer will be immediately available in the field so always bring a killing jar with you. |
 |
| REPAIRING DAMAGED INSECTS: |
| 1. REPAIRING DRIED INSECTS: If you let an insect dry out before pinning it, it will be extremely difficult to pin without damaging the appendages. However, many dried insects will soften if placed into a relaxing jar (a small, sealed jar containing a cotton ball that has been soaked in water). After 48 hours, the dried insect should absorb moisture from the air within the relaxing jar and become somewhat pliable again. |
| 2. REPAIRING DAMAGED APPENDAGES: If an insect's appendage breaks off, carefully glue it back on by touching the broken end of the appendage to a minimal amount of glue and adhering it to the break point. Unfortunately, torn insect wings can not be repaired. |
| |
| PINNING ADULT INSECTS:
|
| Large, hard-bodied insects that you have captured should be preserved by directly pinning them. This entails inserting an insect pin directly through the body of the specimen until the insect rests about one-half inch below the head of the pin (the tallest position on your pinning block). Pins are available in sizes 000 (thin diameter), to 5 (thick diameter) but generally the insect is pinned with the thickest pin it will take without causing damage. Pinning an insect serves the purpose of preserving the specimen for future observation. As such, each insect order needs to be pinned in a location that minimizes damage over time: |
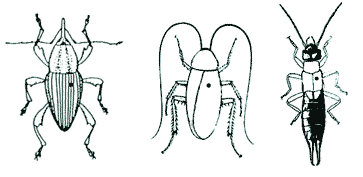
| 1. Adult COLEOPTERA, BLATTARIA, DERMAPTERA & MANTODEA should be pinned through the right tegmen/elytra (hardened wing cover), approximately half-way up the length of the body. |
 |
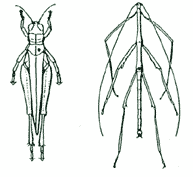
| 2. Adult ORTHOPTERA & PHASMATODEA should be pinned through the metathorax slightly to the right of the mid-line of the body. |
 |
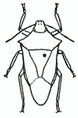
| 3. Adult HEMIPTERA should be pinned through the right corner of the scutellum. |
 |
| 4. Adult DIPTERA, HYMENOPTERA, EPHEMEROPTERA, ODONATA & MECOPTERA should be pinned between the base of the fore-wings, slightly to the right of the mid-line of the body. |
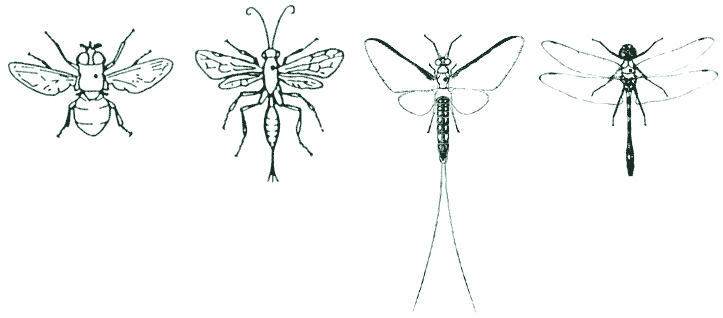 |
| 5. Adult LEPIDOPTERA should be pinned between the base of the fore-wings, with all four wings spread (the hind margin of the fore-wings and front margin of the hind-wings running at right angles to the length of the body). |
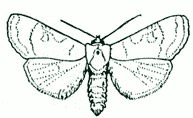 |
| LABELING PINNED INSECTS:
|
| Labeling of pinned insects is required to identify an insect's location and capture date, as well as the type of insect that was captured. This information can be provided on two labels. The first label, which sits closest to the insect (2nd height of your pinning block) should be comprised of 3 lines. The top line provides the PLACE of capture. The middle line provides the DATE of capture (written as day - month - year). The bottom line provides the name of the person that captured the insect. For example: Burnaby, BC 10-Sep-2006 S. Tudent The second label, which sits furthest from the insect (lowest height of your pinning block) should be comprised of two lines. The top line provides the order of the insect on the pin (which you determined using a taxonomic key). The bottom line provides the FAMILY of the insect on the pin (which you determined using a taxonomic key). For example: COLEOPTERA COCCINELIDAE |
 |
| |
| ALCOHOL PRESERVATION OF SOFT-BODIED
INSECTS:
|
| Adult MICROCORYPHIA, THYSANURA, TRICHOPTERA, ISOPTERA, PLECOPTERA, NEUROPTERA & GRYLLOBLATTARIA (as well as larvae & nymphs of all orders) are soft-bodied, and should be preserved within a sealed vial of 80-90% alcohol. Labels should be similar to those of pinned insects but written in pencil (to prevent dissolution), and inserted directly into the alcohol vial with the insect. |
 |
| |
| POINTING/GLUING SMALL INSECTS:
|
| Any adult insect (EXCEPT DIPTERA) that is too small to pin without causing damage should be pointed. Pointing involves inserting an insect pin (usually size 0-1) through the broad end of a small, stout, triangular piece of paper. The small end (tip) of the triangle is bent downward at a right angle (using forceps). A very small amount of glue is placed on this bent tip and this is then applied to the RIGHT side of the insect's thorax. A correctly pointed specimen has its body horizontal to the ground when the pin is upright. Label information and heights should be similar to those of pinned insects however, the pin should be inserted on the right side of the labels so that they are aligned directly beneath the point. |
 |
Any adult DIPTERA that is too small to pin without causing damage should be directly glued to the side of the insect pin. A tiny spot of glue is applied to the insect pin (about one-half inch below the top of the pin), and this is then applied to the RIGHT side of the Dipteran's thorax. Label information, height, and position should be similar to that of pinned insects. |
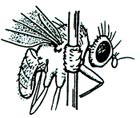 |
| |
| MICROSCOPE SLIDE-MOUNTING INSECTS:
|
| Any insect which is too small to conveniently pin, point or glue needs to be mounted onto a microscope slide for proper identification. A small drop of fixing medium should first be applied to the center of a microscope slide. The insect should then be dropped into the fixing medium, and if possible, legs, and other body appendages should be oriented to allow for easier identification. A second drop of fixing medium should then be placed onto the insect, before lowering an angled coverslip (to remove air bubbles) onto the embedded insect. The slide should be placed on a slide warmer for approximately one week to dry. Label information should be the same as those of pinned insects but should be adhered to the face of the microscope slide, on either side of the embedded specimen. |
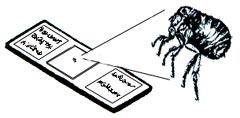 |
| |
 |
| ___________________________________________________________ All images and information appearing on this website are intended for educational purposes only, and are the exclusive property of their respective owners. Website created by Nathan Woodbury |