
|
||
Paramagnetic Transition-Metal Coordination and Organometallic ComplexesParamagnetic organometallic complexes have been much less studied relative to the vast literature on diamagnetic organometallic complexes. We are interested in the synthesis, structural chemistry, reactivity and, of course, magnetic behaviour of this underexamined class of compounds. To what extent does the presence of unpaired electrons at the metal centre affect the stability, structure and reactivity of these compounds? Reactivity studies in particular with respect to catalysis and redox chemistry are explored.
We have developed a series of diamido ligands of the form {[RN(SiMe2)]2O}2-, termed [RNON]2- (R=alkyl, aryl), which incorporate a neutral silylether donor. The ether donor acts as a gated stabilizing agent, coordinating only when the metal centre requires additional electron-density. Selected ongoing projects with this class of ligands and paramagnetic metal centres are described below. Quantum spin-admixed iron(III) complexesWhen Li2[RNON] (R=tBu) is reacted with iron(III), an extremely rare "quantum-spin-admixed" spin state material is formed, which has implications in bio-inorganic chemistry due to the importance of iron-containing proteins and their spin-states in nature. The Mossbauer spectrum of this compound shows an extremely wide doublet from 4-300 K, a signature for this spin-admixed spin state.  Upon using arylamides instead of R = tBu to probe the influence of the amido-R group on the spin-state, unprecedented tetrahedral iron(III) "ate" complexes stabilized by strong Li-π interactions were generated, which are high-spin systems; this result was featured on the cover of Chemistry: A European Journal. Currently, we are altering the ligand's electronic and steric profile in an effort to understand the factors controlling the rare spin-admixed state. This information is vital to comprehending the reactivity of metalloproteins or paramagnetic catalysts. Collaborative DFT calculations are aimed at understanding the underlying orbital descriptions of observed spin-states. 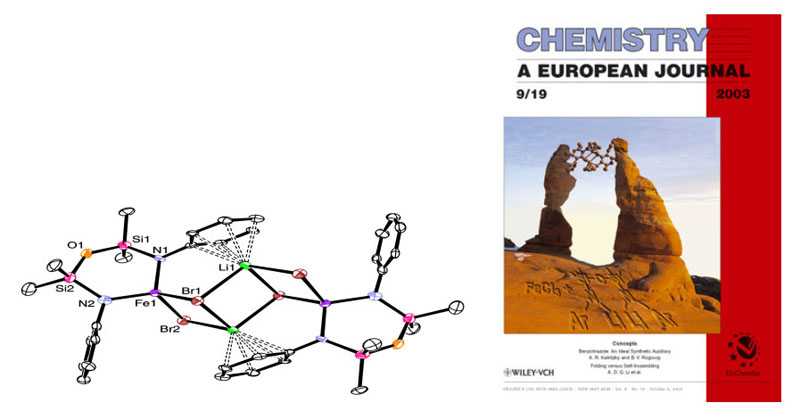 For more details, see:Mund, G.; Batchelor, R. J.; Sharma, R.D.; Jones, C.H.W.; Leznoff, D.B. "{FeCl[tBuN(SiMe2)]2O]2: The first multinuclear Iron(III) System Exhibiting Spin-Admixture." J. Chem. Soc, Dalton Trans., 2002, 136-137. Download Mund, G.; Vidovic, D.; Batchelor, R.J.; Britten, J.F.; Sharma, R.D.; Jones, C.H.W.; Leznoff, D.B. "Unusual iron "ate" complexes stabilized by Li-π interactions." Chem. Eur. J., 2003, 9, 4757-4763. Download Synthesis and reactivity of paramagnetic metal amido complexesIn addition to standard metathesis methodology to synthesize metal amido complexes, we have employed a more unusual route that uses an yttrium(III) bis-amido complex as a mild ligand transfer agent. Using this route, we have been able to prepare previously unattainable chromium(III) diamidoether complexes, which have potential applications as alkene polymerization catalysts. 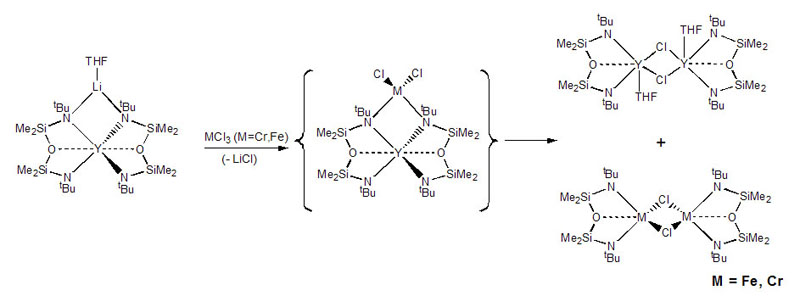 Scheme: Amido yttrium(III)-mediated ligand transfer route to other metal(III) amido complexes and the heterobimetallic intermediate. The reactivity of classical non-chelated M[NR2]x complexes is dominated by decomposition or amide-transfer reactions. In addition to their unusual structures and magnetic properties, our paramagnetic chelating metal amido complexes should show a range of unusual reactivities due to the greater inherent stability of the ancillary ligand, and we are exploring the reactivity of our complexes with respect to ligand substitution, redox chemistry, binding of neutral donors and small molecule activation. For example, the reaction of [tBuNON]FeCl with LiNPh2 yields a reduced iron(II) product with an unusual bridging [NON] ligand (shown below). 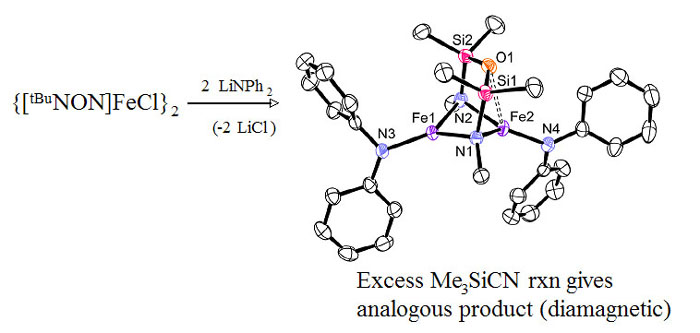 Related projects in the Leznoff group include pursuing paramagnetic (a) high-valent transition metal organometallics, (b) early/late heterobimetallic complexes and their unique reactivity and (c) mixed-valent complexes and their unusual magnetic and electronic properties. For more details, see:Leznoff, D.B.; Mund, G.; Jantunen, K.C.; Bhatia, P.; Gabert, A.J.; Batchelor, R.J. "Diamidoether Complexes of Uranium and Transition-Metals." J. Nucl. Sci. Tech., Supplement 3, Nov. 2002, 406-409. Mund, G.; Vidovic, D.; Batchelor, R.J.; Britten, J.F.; Sharma, R.D.; Jones, C.H.W.; Leznoff, D.B. "Unusual iron "ate" complexes stabilized by Li-π interactions." Chem. Eur. J., 2003, 9, 4757-4763. Download Wong, E.; Das, A.K; Katz, M.J.; Nishimura, Y.; Onishi, M.; Batchelor, R.J.; Leznoff, D.B. "Diamidoether complexes of Yttrium(III) and Chromium(III): Synthetic challenges and surprises" Inorg. Chim. Acta, 2006, in press (Special Issue in honour of B.R. James). Ligand design and synthesis for use with paramagnetic metal centres
Mixed-donor ligands generate electronically non-symmetrical reaction sites, but are often difficult to prepare, or require multiple synthetic steps. Our recently reported high-yield, simple one-step synthesis of an amido-amino-siloxo [NN'O]2- ligand by an in situ 1,3-silyl rearrangement has opened the door to exploring the coordination chemistry of a series of very unsymmetrical amido donor ligands and comparing their chemistry with the more symmetrical diamidoether ligands. 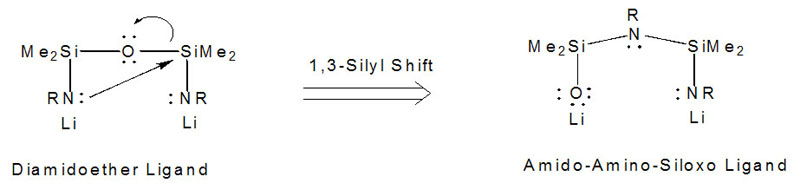 As an example of how important the ligand design can be, the reaction of chromium(II) with Li2[RNON] and Li2[RNN'O] yields very different products: an amido-bridged dimer in the former, and a siloxo-bridged dimer in the latter. In fact, when R is very bulky, no stable product with [RNON] and chromium(II) forms, but with [RNN'O], a very electron-deficient three-coordinate chromium(II) dimer is isolated, which is so "hot" that it activates the aryl-ring of the ligand. Such interactions may help to stabilize the resting state of amido-supported metal complexes that are active in alkene polymerization and we are exploring a range of chemistry with these ligands and paramagnetic transition-metals. 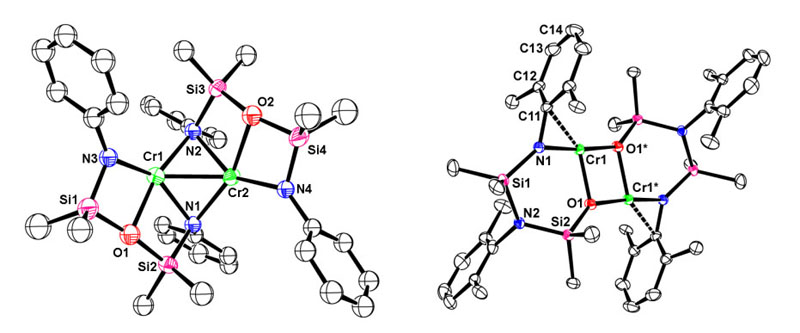 Figure: What a difference a ligand rearrangement makes! [RNON]Cr dimer with bridging amides and a Cr-Cr bond (left) and [RNN'O]Cr dimer with bridging siloxides, no Cr-Cr bond and an activated arylamide ring. For more details, see:Mund, G.; Gabert, A.J.; Batchelor, R.J.; Britten, J.F.; Leznoff, D.B. "A rare ether-bridged cobalt complex which gives rise to an unusual 'serpentine' metal-ligand binding motif." J. Chem. Soc., Chem. Commun., 2002, 2990-2991. Download Haftbaradaran, F.; Mund, G.; Batchelor, R.J.; Britten, J.F.; Leznoff, D.B. " Synthesis of mixed-donor amido/amino/siloxo ligands from symmetrical diamidosilylether ligands via a retro-Brook rearrangement and their chromium(II) complexes" Dalton Trans., 2005, 2343-2345. Download Back to the research page. |
||
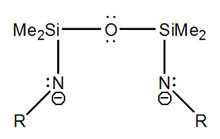 Ligand design and synthesis are an inherent part of this project. The Leznoff group uses amido (NR2-) donors, which are versatile ligands due to their ease of steric and electronic modification (via the nitrogen substituent) and are ideal for the stabilization of high-oxidation state metal centres due to their strong pi-donating ability. In particular, multidentate amido ligands have been shown to be excellent for the stabilization of high-valent diamagnetic organometallic systems, such as for Zr(IV) and Ti(IV)-based alkene polymerization catalysts. However, chelating diamido ligands have rarely been used with paramagnetic first-row transition metals despite the expectation of very different chemistry compared with the classical monodentate M[NR2]x system.
Ligand design and synthesis are an inherent part of this project. The Leznoff group uses amido (NR2-) donors, which are versatile ligands due to their ease of steric and electronic modification (via the nitrogen substituent) and are ideal for the stabilization of high-oxidation state metal centres due to their strong pi-donating ability. In particular, multidentate amido ligands have been shown to be excellent for the stabilization of high-valent diamagnetic organometallic systems, such as for Zr(IV) and Ti(IV)-based alkene polymerization catalysts. However, chelating diamido ligands have rarely been used with paramagnetic first-row transition metals despite the expectation of very different chemistry compared with the classical monodentate M[NR2]x system.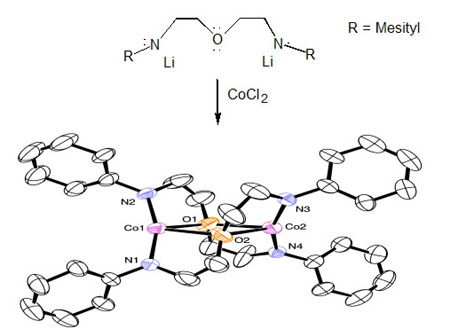 The design of new ligands and their impact on the structure and reactivity of a metal complex is central to inorganic chemistry. As we showed with the tBu- and aryl-subsitituted [RNON]Fe complexes, the structures and magnetic properties changed drastically with a change in R-group; this has important implications in bio-inorganic chemistry. In order to probe the importance of the silylether backbone on the observed chemistry, we are exploring compounds of paramagnetic transition-metals with a related diethylether backbone. Although it looks quite similar to the silylether analogue, this flexible carbon-[NON] ligand generates an unusual complex in which the cobalt atoms of the dimer are bridged in a ‘serpentine’ fashion through the ether moieties of the ligand rather than the stronger amido donors. This is the first example of an ether-bridged metal complex. The coordination chemistry of this type of ligand and the reactivity of the unusual resulting metal complexes are of interest, particularly since this new binding motif may have implications for the use of diamidodonor ligands in alkene polymerization catalyst design.
The design of new ligands and their impact on the structure and reactivity of a metal complex is central to inorganic chemistry. As we showed with the tBu- and aryl-subsitituted [RNON]Fe complexes, the structures and magnetic properties changed drastically with a change in R-group; this has important implications in bio-inorganic chemistry. In order to probe the importance of the silylether backbone on the observed chemistry, we are exploring compounds of paramagnetic transition-metals with a related diethylether backbone. Although it looks quite similar to the silylether analogue, this flexible carbon-[NON] ligand generates an unusual complex in which the cobalt atoms of the dimer are bridged in a ‘serpentine’ fashion through the ether moieties of the ligand rather than the stronger amido donors. This is the first example of an ether-bridged metal complex. The coordination chemistry of this type of ligand and the reactivity of the unusual resulting metal complexes are of interest, particularly since this new binding motif may have implications for the use of diamidodonor ligands in alkene polymerization catalyst design.